Arrhenius And Bronsted Lowry Acids Bases Worksheet
Worksheet bronsted lowry acids and bases. And since ammonia does not contain hydroxide ions, i.e.

Arrhenius vs BronstedLowry Acids & Bases YouTube
Describe and name acids and bases.
Arrhenius and bronsted lowry acids bases worksheet. Chapter 10 acids and bases i. Later, we extended the definition of an acid or a base using the. Bronsted lowry acids and bases worksheet.
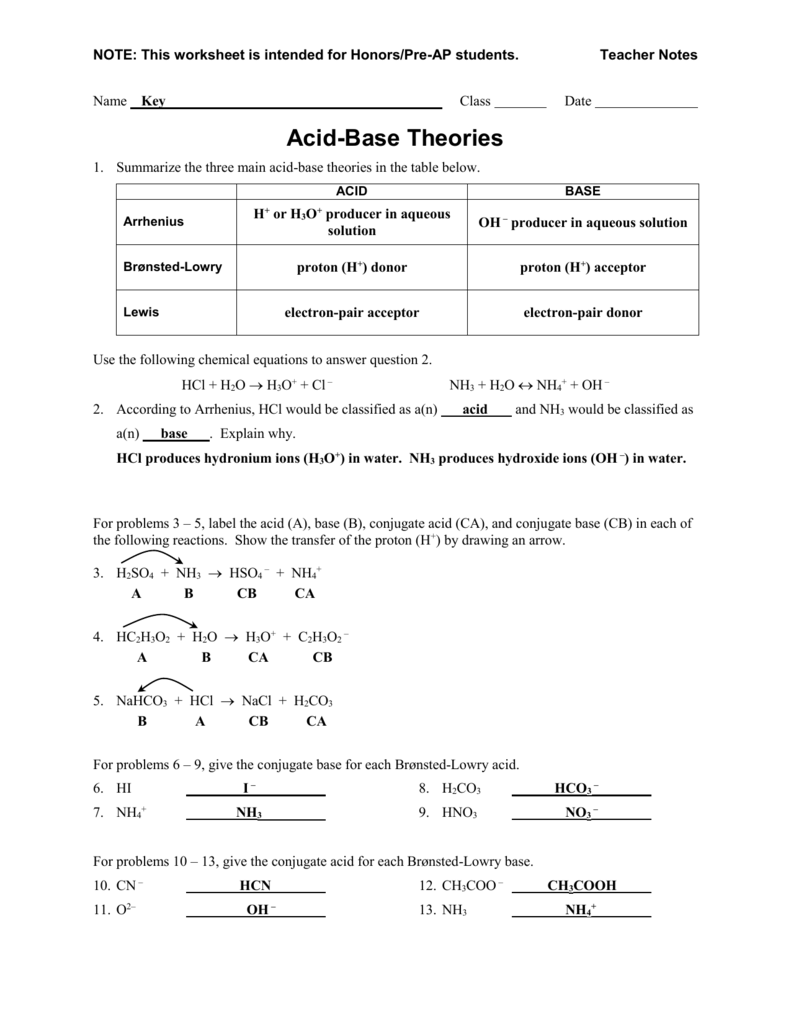
Brønsted lowry acids and bases salisbury university. An arrhenius base is a substance that when added to water increases the concentration of. Bases taste bitter, feel slippery, and neutralize acids.
The chemical formulas of arrhenius acids are written with the acidic hydrogens first. The arrhenius definition of acids and bases is one of the oldest. Brønsted lowry acids and bases github pages.
Bronsted lowry acids and bases by. Bronsted theory of acids amp bases help teaching. Bronsted lowry acids and bases worksheet answer key.
Bronsted lowry acid acetate scribd. Arrhenius defined an acid as a compound that increases the concentration of hydrogen ion (h +) in aqueous solution.many acids are simple compounds that release a hydrogen. The bronsted lowry and lewis definition of acids and bases.
Acids were compounds with hydrogen that ionized when aqueous to form h+. 1 inspiring women's civil rights that have fought for equality 2 the acquisition of capybara of argentina shows why our environment needs to rehabilitate 3 which difference between a villa and a house? What is the brønsted lowry equation yahoo answers.
The swedish chemist svante arrhenius developed the first chemical definitions of acids and bases in the late 1800s. The brønsted lowry definition of acids and bases liberates the acid base concept from its limitation to aqueous solutions as well as the requirement that bases contain the hydroxyl group. Arrhenius and bronsted lowry acids bases worksheet.
Adds h+ to solution when ionized. A base accepts a proton to form a conjugate acid. Acids and bases lewis vs bronsted.
An acid is a proton donor and a base is a proton acceptor. 4 what is considered dangerously high arterial pressure? Bronsted lowry answers bing blog with pdf links.
Is nh3 an arrhenius base? Unit 12 quiz bronsted lowry theory and ka values. Is nh3 an arrhenius base?
Typing drawing or uploading one. Bronsted lowry acids the bronsted lowry acid base concept journal of chemical. Bronsted lowry theory of acids and bases answer key.
Acids taste sour, may sting, and neutralize bases. An acid is a proton donor and a base is a proton acceptor. The only difference is that the solution does not have to be water.
An arrhenius acid is a substance that when added to water increases the concentration of h+ ions present. A base accepts a proton to form a conjugate acid. In an earlier chapter on chemical reactions, we defined acids and bases as carl axel arrhenius did:
Bronsted lowry acids amp bases worksheet. We can still refer to the exact same acids as listed for the arrhenius acid examples, but this time we'll change the solvent to ammonia, alcohol, or anything else. We identified an acid as a compound that dissolves in water to yield hydronium (h 3 o +) ions and a base as a compound that dissolves in water to yield hydroxide ions (oh −).however, this definition is somewhat limited.
Bronstedlowry Acids and Bases Worksheet
Bronstedlowry Acids and Bases Worksheet
Bronsted Lowry Acids And Bases Worksheet Bronstedlowry

Quiz & Worksheet BronstedLowry and Lewis Definition of
Bronsted Lowry Acids And Bases Worksheet Bronstedlowry
Bronstedlowry Acids and Bases Worksheet
Bronstedlowry Acids and Bases Worksheet
Bronstedlowry Acids and Bases Worksheet
Bronstedlowry Acids and Bases Worksheet
Bronstedlowry Acids and Bases Worksheet

Worksheet 42 Bronsted Acids and Equilibria
Bronstedlowry Acids and Bases Worksheet

Solved Worksheet 18 Acids And Bases The BronstedLowry
Bronstedlowry Acids and Bases Worksheet
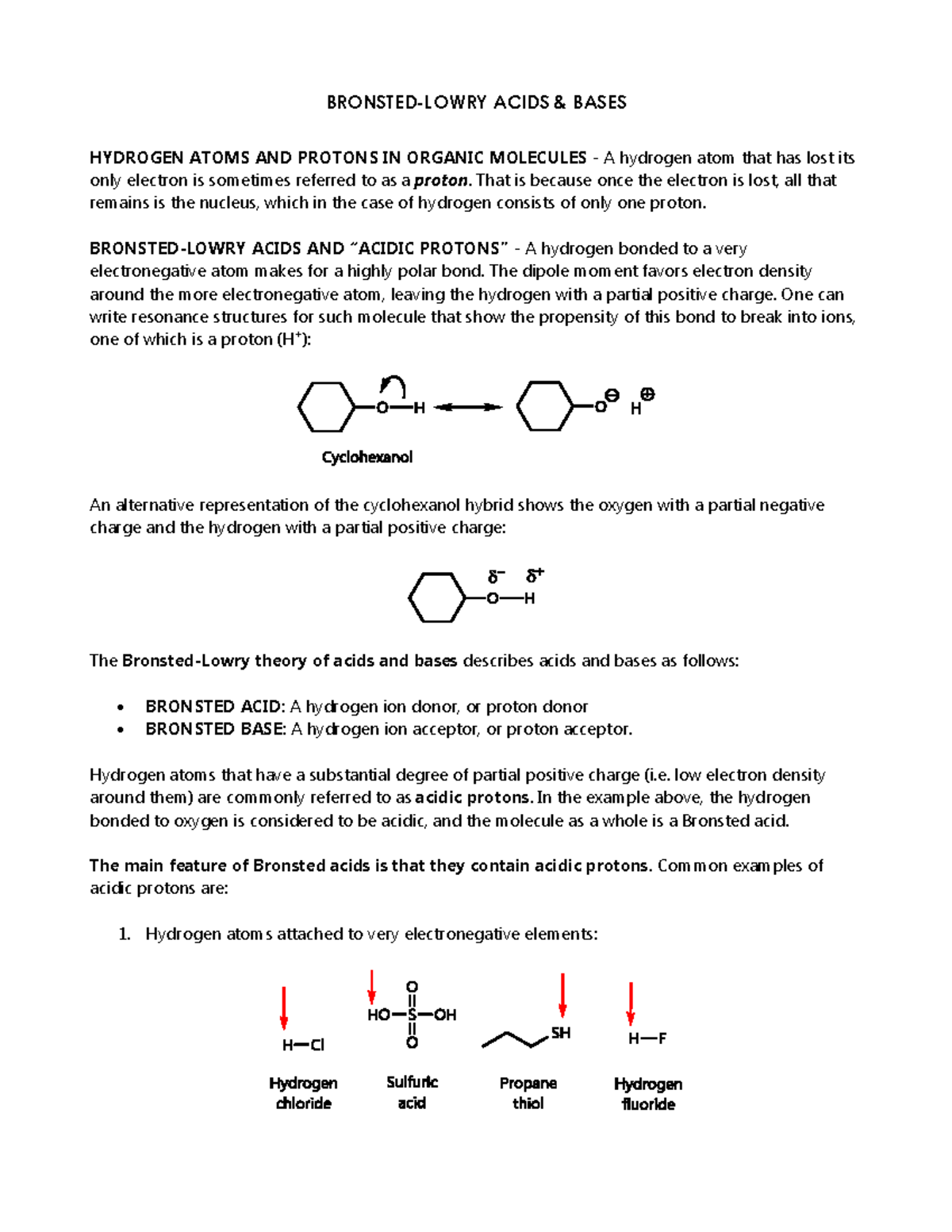
BronstedLowry Acids & Bases StuDocu